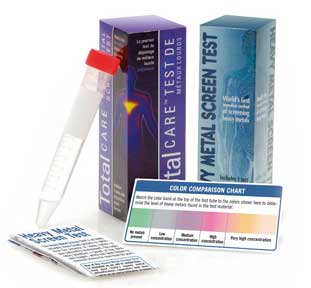
TotalCARE Heavy Metal
Test Kit
tests drinking water,
saliva, urine and many
materials in your environment for heavy metal
toxicity
Order Now
Heavy
Metal Quick Test Instructions
Heavy Metal
Quick Test Product Description
The truth will set you free, free of the
debilitating effects of heavy metal overload within
our tissues. Don't believe the old adage that what
you do not know won't hurt you.
The truth is, what we don't know may well be what is
hurting us, or at least, keeping us from feeling our
best. The facts are that we live with
environmental pollution and heavy metal
contamination, but most people do not realize how it is affecting
them directly. Now you can find out with our Heavy Metal Screen Tests.
What Can You Test:
Our Heavy Metal Screen Test
allows you to instantly test your drinking water,
yourself, urine and many materials in your
environment for heavy metal toxicity. Suspect paint
has lead, test it. Think there is lead in your
crystal glasses? Test them. If you want to know how
well your body is able to cope with heavy metal
ions, now you can know. You can even learn about the
heavy metal content of the foods
you eat. Isn't that great? At home, at work,
at the cottage, anywhere TotalCARE Heavy Metal
Screen Test gives you the tools to take charge of
you environment and your health. You no longer have
to be a victim or a statistic.
Why should you test
for the presence of heavy metals?
Heavy Metal
Toxicity is directly or indirectly linked to issues
many people suffer regularly:
-
Headaches
-
Anger,
irritability, depression
-
High blood
pressure, high cholesterol
-
Lack of
energy/listlessness
-
Weight
gain
-
Difficulty
concentrating and/or remembering
-
Rashes,
allergic reactions or sensitivity
-
Bloating,
gas, or constipation
Heavy metals also have been found to contribute to
-
Arthritis
-
Asthma
-
Chronic
fatigue
-
Diabetes
-
Fibromyalgia
-
Heart
disease, arterial sclerosis
-
Multiple
sclerosis
-
Parkinsons
disease
-
Ulcers,
and many others
Used in conjunction with
Bio-chelat, TotalCARE Heavy
Metal Screen Test empowers you to free yourself from
the negative effects of metal toxins and to prevent
even greater health challenges in the future.
TotalCARE Heavy Metal Screen Test Is Easy To
Use
TotalCARE Heavy Metal Screen Test is painless, easy
to do and produces quick easy-to understand results.
Developed by naturopathic doctor and specialist in
environmental health, Thomas Nissen, TotalCARE Heavy
Metal Screen Test is based on sound scientific
principles and 20 years of research.

Heavy Metal Screen
Test 1-2 Description
These screen test kits are designed for use at home by the
general public as well for therapists. They allow you to identify the level of
Heavy Metal concentration in your body. The procedure is simple, cost effective,
and completed within 5minutes.
The Heavy Metal Screen Test 1-2 measure semi-quantitatively the elimination of
free zinc, copper and lead ions in the urine. Mercury, cadmium, nickel, cobalt
and manganese ions are also detected. These, however, play only a minor role in
the elimination of the urine, due to their habitual lower concentrations. With
the help of the color chart you will be able to identify the degree of the metal
concentration as well your capacity of detoxification:
- No metals present
- Low concentration
- Medium concentration
- High concentration
If your self-test shows the presence of free heavy metals,
all sources of contamination should be eliminated and a detoxification process
should be undertaken.
In conjunction with Bio-Chelat
Defense™, the home test kit empowers you to free yourself from the negative
effects of metal toxins and can prevent even greater health challenges.

Continuing to offer the best quality to our clients, in
2010-2011, naturopathic doctor Thomas Nissen and chemist Günter Bienfait from
Germany improved this screen test with chemist Mathias Del Duca and Dr. med.
Gers Hitl. They enhanced the precision and the stability to determine the shelf
life and the interfering factors that caused variations to the quality of the
product.
The information the kit provides is for general educational purposes only. The
test is not intended to replace advice from a competent and knowledgeable
healthcare professional. If you are experiencing serious symptoms (nausea,
vomiting, headaches, sweating, difficulty breathing, convulsions, trembling) or
you believe you have acute heavy metal poisoning, contact your health care
provider immediately. Seek qualified healthcare advice for the treatment of any
illness or disease.

For
additional information we encourage you to
read this article concerning heavy metal toxicity to
educate yourself on this important issue.
"It no longer matters what you call your disease.
The label your doctor gives you is meaningless. What
matters is what caused it. The lowering of huge
amount of hidden heavy metals has turned around the
worst heart diseases, improved memory, mood and IQ.
It is one of the most important decisions of your
life.” Dr. Sherry Rogers, MD. Author of the book: ”Detoxify
or Die.”
Numerous scientists worldwide are supporting the
view today that all life processes are being
determined by subtle electromagnetic and photon
phenomena [see Prof. Dr. A. Popp,, Dr. Voll (EAP),
Dr. Dr. Schimmel (Vega System) and many more). All
electrically active metals (ions) and particularly
heavy metals can disturb the harmony of the
electromagnetic and photon energies in the body,
causing disharmony and disease. They also can
increase the production of free radicals
million-fold.
It has been stated that 90% of all chronic and
serious illnesses could be prevented if we were able
to eliminate the 600 most dangerous environmental
toxins (Dr. J. Higgensen, Head of Cancer Research,
WHO, Geneva, Switzerland). Every health practitioner
is fully aware of the devastating influence heavy
metals and/or ionic metals can have on our mental,
emotional and physical health and well-being.
Until recently, most health care professionals and
researchers assumed that heavy metals had to be
taken into account only when a patient showed
definite symptoms of 'poisoning'. We realize now
that our health and well-being is affected by much
lower levels of heavy metals than previously
assumed. Health authorities constantly correct
'permissible' maximum levels downwards.
It is becoming more difficult to accurately
determine the appropriate drug profile in a given
case, because the respective simile of symptoms has
undergone a shift due to the presence of heavy metal
ions. In fact, this phenomenon may be observed for
the majority of the classic Hahnemann remedy
profiles and it is fair to say that at the present
time the effectiveness of any antioxidant therapy is
significantly compromised by the presence of heavy
metal ions. It is therefore important to first
identify the heavy metal in question and then the
degree of its involvement. Then, as the cause of the
condition, the heavy metal ions must be removed and
cleared out.
In cases of acute heavy metal poisoning (commonly
the result of accidents or extreme workplace-related
contamination), clinical toxicology is generally
able to provide an effective, quick response with
the DMPS procedure administered as mobilization test
and antidote. However, hardly any appropriate
treatment or diagnostic procedure is available for
cases of long-term heavy metal contamination. No
satisfactory method exists for the early recognition
of heavy metal contamination.
Two Types of Metals
The methods used to detect heavy metal contamination
are cumbersome and costly and in some instances
can’t differentiate between organically bound and
free metal atoms (e.g. Cu, Zn in spectrometric
analyses). Recent research has shown that it is
essentially electrically active heavy metal atoms
not bound with organic complexes that actively
destroy molecular compounds and thereby cause the
formation of free radicals. Up to a certain point, a
healthy body is able to bind (i.e. chelate) free
heavy metal atoms, i.e. neutralize their
electromagnetic charge and clear them out. If this
mechanism is no longer able to function because too
many toxins have accumulated in the organism, the
number of free radicals will increase, especially if
the body is suffering an antioxidant deficiency at
the same time. In such cases, administering
antioxidant supplements will not solve the real
problem, namely the accumulation of heavy metal ion
deposits in the body.
Better Than Hair and Blood Analyses
Unfortunately, traditional methods like hair or
blood analyses are not able to uncover these
connections for the simple reason that the organic
sample is destroyed in the course of the analysis.
Such procedures are therefore unable to
differentiate between metal atoms bound with organic
complexes and unbound and therefore
electro-magnetically active ions, a difference that
is crucial in the assessment of the overall
situation.
A new way to assess heavy metals
In 1925 Helmut Fischer of the Siemens Concern in
Berlin succeeded in detecting heavy metal ions by
means of a dithizone process. As a reagent,
dithizone is able to indicate the presence of heavy
metal ions in qualitative and in quantitative terms.
In binding with them, colored complexes are formed
in the interior of the molecule which are soluble in
non polar organic solvents. The coloration of these
solutions is very intensive, its particular
coloration determined by the atomic radius of the
respective metal present in the complex. The
reaction times of the heavy metal ions vary;
therefore, depending on their respective
concentrations, different colorations may occur from
which one can, in addition to the qualitative
conclusions (the dithizon reagent binds to Cu, Zn,
Cd, Hg, Pb, Mn, Co, Ni,), draw also quantitative
ones regarding the contaminant. (At the lower ppm
level, even at the ppb level).
The dithizone heavy metal reagent allows the
detection of free heavy metal ions in bodily liquids
like urine and saliva. By administering the test
reagent as an exploratory measure, contaminations
from amalgam fillings or from the environment
(cadmium, lead, zinc, copper, manganese, nickel and
cobalt - pointing to infections, organ or system
disorders), as well as potential health problems,
can be identified on the spot. The need for
detoxification is established before any specific
therapy is administered. The test reagent is
therefore an important and recommended aid during
the initial evaluation. As it is urgent that
necessary counter-measures be implemented in the
patients’ detoxification therapy, a method to expose
and monitor heavy metals becomes crucial.
The dithizone reagent can also be used to determine
the environmental sources of the contamination in
aqueous solutions such as tap water. Since all heavy
metal ions are water soluble, solids like food
items, porcelain dishes, dust samples from carpets,
wall paints and wall paper etc. can be tested for
heavy metals by soaking them in distilled water
beforehand. In other words, in addition to being a
diagnostic tool for urine and saliva, the reagent is
also useful in finding contaminants in the patient's
environment.
Replacement Reaction or How to Asses Heavy Metal
Toxicity
The sheep study done at the University of Calgary in
Canada (sheep had amalgam fillings placed in their
mouths) clearly showed that very little mercury is
found in the urine and in the blood, but highest
amount are shown in the kidneys, stomach and other
organs. Since this is the case, how is it possible
to assess mercury or other heavy metal toxicity via
the urine?
To understand this, a short review of basic
bio-chemistry and how heavy metals react in the body
is necessary.
In the human system, the bivalent metals are engaged
in a continuous fight against one another, e.g.
copper against zinc, magnesium against calcium,
which results in the replacement of the "lighter"
element by the "heavier" one in terms of their
atomic masses. Replacement reactions, also called
“fight for the site”, occur when heavy metals grab
the biological spaces that should be filled by
necessary minerals.
Just as carbon monoxide replaces essential oxygen,
other elements and compounds cause their toxic
effect by replacing chemicals essential to the body
functions. Within a group, for example group 2 in
the periodic table of elements (2 refers to the
number of extra electron) there is zinc (Zn),
cadmium (Cd), and mercury (Hg), in order of
increasing atomic weight. (65, 112, and 200
respectively). Zinc in its ionic form, Zn2+, is
necessary for proper body function, although an
excess is toxic. Cadmium, found in paints,
cigarettes, tires, and brakes, is toxic. Mercury,
found in amalgam fillings, paints, and some
industrial processes, has no known use in the body
and is even more poisonous.
Since cadmium and mercury, in their more soluble
ionized or salt forms, will attempt to participate
in the same biochemical reactions as zinc, their
presence will prevent the zinc reacting and
performing its functions in the body. This is like a
65 pound person (zinc) competing unsuccessfully with
112 pound (cadmium) and 200 pound (mercury) people
in a wrestling match.
In vivo and ex vivo displacement of zinc from
metallothionein by cadmium and by mercury.
Divalent cadmium and mercury ions are capable in
vitro of displacement of zinc from metallothionein.
This process has now been studied in vivo and ex
vivo, using the isolated perfused rat liver system,
in order to determine if this process can occur in
the intact cell. Rats with normal and elevated (via
preinduction with zinc) levels of hepatic zinc
thionein were studied. Cd(II) completely displaces
zinc from normal levels of metallothionein and on a
one-to-one basis from elevated levels of
metallothionein, both in vivo and ex vivo. Hg(II)
displaces zinc from metallothionein (normal or
elevated) rather poorly, as compared with Cd(II), in
vivo, probably due to the kidneys preference for
absorbing this metal. Ex vivo Hg(II) displaces zinc
from metallothionein (normal or elevated) on a
one-to-one basis, with considerably more mercury
being incorporated into the protein than in vivo.
The results of double-label ex vivo experiments
using metal and [35S]cysteine (+/- cycloheximide)
were consistent with the above experiments,
indicating that de novo thionein synthesis was not
required for short term incorporation of cadmium and
mercury into metallothionein. These data are
supportive of the hypothesis that cadmium and
mercury incorporation into rat hepatic
metallothionein during the first few hours after
exposure to these metals can occur primarily by
displacement of zinc from pre-existing zinc thionein
by a process which does not require new protein
synthesis.
Chem Biol Interact. 1984 Jul;50(2):159-74
As a result, mercury leaching into the body from
silver-mercury amalgam fillings, or lead and cadmium
absorbed through food, will cause symptoms of zinc
deficiency such as fatigue, PMS, thyroid problem,
loss of smell and taste, macular degeneration,
prostate enlargement, rheumatoid arthritis,
sterility, immune suppression, etc., even if there
is plenty of zinc available.
Other symptoms caused by mineral deficiency and
displacement by a heavy metal (Hg, Cd, Pb,) include:
-
Magnesium Irregular heartbeat, osteoporosis, receding gums, etc
-
Iron Anaemia
-
Copper Anaemia,
Thyroid dysfunction, impaired digestion,
scoliosis
-
Zinc Anorexia
nervosa, loss of taste, low libido, PMS,
etc
-
Iodine Thyroid
dysfunction
Heavy Metals
Cause Toxic Accumulation of Essential Minerals
By taking the biological spaces of the essential
minerals, heavy metals are blocking the absorption
of essential minerals and simultaneously a toxic
accumulation of unbound zinc and copper ions occurs.
At this stage of toxic contamination, the discharge
of copper and zinc ions from the organism is not yet
relevant, but as free electrically active metals,
they can be made visible with the dithizone reagent.
The valuable essential metals copper and zinc have,
in effect, become toxic metals. Diagnostically, the
test indicates that the body cannot handle the heavy
metals and uses liver, kidneys and other tissue as
waste deposit sites.
Therefore when checking the urine for heavy metals
by using the dithizone reagent, toxic amounts of
copper and zinc (direct antagonist to most of the
heavy metals which have all a 2+plus valence) will
always show up first during the test procedure and
indicate the presence of heavy metals in the body.
Other reasons why the amount of unbound heavy metal
ions in the urine is very high are:
1. Excessive intake of supplements.
2. Metals are accumulating due to constipation or
reduced bile flow: Heavy metals and in particular
mercury accumulates in the gallbladder, creating a
perfect environment for bacteria and fungus which
leads to a sluggish or thick bile.
Conclusion
In a healthy body with a functioning detoxification
system or in the absence of heavy metals, there
should be no free heavy metal ions found in the
urine. Consequently, the more unbound metal ions are
found in the urine, the more the body’s
detoxification capacities are exhausted.
Instead of measuring the mercury or other heavy
metal ions, which is very difficult to assess, since
these heavy metals are neither in the blood nor in
the urine, the indirect disturbance caused by the
heavy metal atoms are measured.
Since heavy metals contribute to up to 80% of the
causes of all diseases, the assessment for heavy
metal contamination has become an essential
component of any initial diagnosis. The dithizone
reagent offers an alternative way to assess heavy
metal toxicity and is actually the only test which
allows the assessment on the intracellular level.
Dithizone References:
1. Isolation and Determination of Traces of Metals.
The Dithizone System. H.J. Wichmann, Food and Drug
Administration, U.S. Department of Agriculture,
Washington, D.C; Industrial and Engineering
Chemistry.;
2. Journal of Industrial Hygiene and Toxicology
Vol.29, No.3, May, 1947; A comparative Study of The
Lead Content Of Street Dirt in New York City in 1924
and 1934.;
3. Kaye, Sidney: A study of the analytical methods
for the determination of lead from biologic
materials, with special emphasis on the dithizone
method. M.Sc. thesis, New York University 1939;
4. : Chem Biol Interact. 1984 Jul;50(2):159-74
5. Clinical Neuropathy, Vol.15 No. 3-1996(139-144)

|
Order Heavy Metal Quick Test |
|

Shipping Information
What's the
difference between the TotalCare Test and the Heavy
Metal Screen Test?
The test kits shown in image above are the same kits
made by the same manufacturer. The TotalCare test
kit has two tests, while the Heavy Metal Screen test
has only one, so when you order one we send you the
Heavy Metal Screen test. When you order two we send
you the TotalCare test kit or two of the Heavy Metal
Screen tests, and when you order three
we send you either 3 Heavey Metal Screen test kits
or one plus a TotalCare Heavh Matal test kit.
Out of stock: Do Not Order:
Call Us First
|
1 Heavy Metal
Quick Test Kit $17.95 |
$15.95 |
|
|
2 Heavy Metal
Quick Test Kit $14.95 each |
$29.90 |
|
|
3 Heavy
Metal Quick Test Kit $13.95 each |
$41.85 |
|
|
In
Stock |
|
Heavy
Metal Quick Test Instructions
|
Return to Testing Kit Page
pHion pH
Test Strips |
Chlorine Test Kit |
Fluoride/Chlorine Test Kit
WaterSafe
City Water Test Kit |
WaterSafe
Well Water Test Kit |
Alkaline Water Test Kit
Heavy Metal Test Kit |
Hanna ORP
Test Meter | Hanna ORP/Temp/pH Test Meter
All Replacement Filter Cartridges
All Prefilter and Housing Units
Heavy
Metal Quick Test Instructions
Health authorities
(WHO 1974, Florence, Italy) estimate that at least
90% of all chronic diseases can be attributed to
environmental pollution in one way or another. Heavy
Metals are the major source for the production of
free radicals as well as undermining the internal
environment and body chemistry. Heavy Metals reduce
the efficacy of medical treatment by up to 60%.
There is little hope for antioxidants and mineral
supplements to do their job properly, if the body is
burdened with heavy metals!
The scientifically documented Heavy Metal
Test (Screen) allows the detection of free
electrically active heavy metal ions in an aqueous
solution by means of a simple procedure and in just
a few minutes. This exploratory procedure, employed
as an in vitro screen tool, is based on the
dithizone(1) reaction method which has been known to
chemical science for more than 60 years.
(1) Isolation and Determination of Traces of Metals.
The Dithizone System. H.J. Wichmann, Food and Drug
Administration, U.S. Department of Agriculture,
Washington, D.C; Industrial and Engineering
Chemistry
Heavy Metal Test: Screening
Instructions
 |
Open test-tube and place one of the
small square Test Paper into test tube
solution.
|
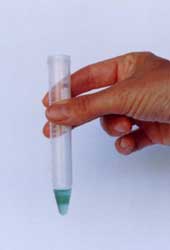 |
Close test tube and shake gently until
solution turns green. (Within 30 –60
seconds)
|
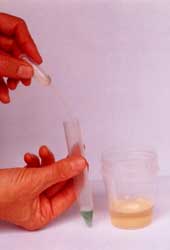 |
Then add 5 ml with the pipette of the
liquid to be tested e.g. (water, saliva,
urine,) into the test-tube. (The
test-tube is now filled up to the 6ml
line) Shake vigorously for 15-30
seconds, stop and allow the solution to
react for 1 minute.
|
Evaluation of Heavy Metal Screen
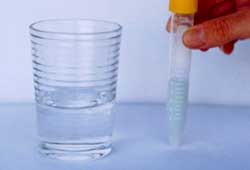 |
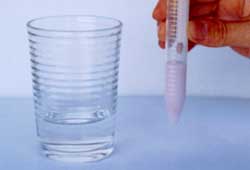
Water Test
Color remains green: No heavy metal ions
|
 |
Water Test
Distinctive color: Purple, pink, beige,
red. High heavy metal concentration (ca.
5ppm-3ppm)
|
 |
Urine Test
Color remains green: No heavy metal ions |
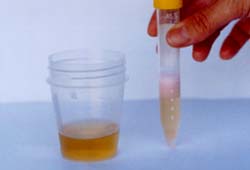 |
Urine Test
Distinctive color: Purple, pink, beige, red.
High heavy metal concentration (ca.
5ppm-3ppm)
|
TEST INTERPRETATION
Any color change
deviating from green viewed directly below the
surface level of the test tube represents the
presence of free or unbound metal ions (Cu, Zn, Cd,
Hg, Pb, Mn, Co, or Ni).
Other Heavy Metal Links
[Top]
[Top]
The statements enclosed herein have not been
evaluated by the Food and Drug
Administration, Canadian or Mexican health
authorities. The products mentioned on
this site are not intended to diagnose,
treat, cure, or prevent any disease.
Information and statements made are for
education purposes and are not intended to
replace the advice of your family doctor.

Product Categories:
Far Infrared |
Exercise
|
Sleep Aid
| Water
Filtration
|
Air Filtration
| Test Kits
|
Supplements
|
Books
| EMF
|
Juicers
|
Skin Care
|
911
Home |
Privacy Policy |
Return
Policy |
Disclaimer
|
Contact Us
Copyright 2018
, MicroWaterMan.com, All Rights Reserved
[top
of page]
|



































 Reduce Electric Bill & EMF Pollution
Reduce Electric Bill & EMF Pollution